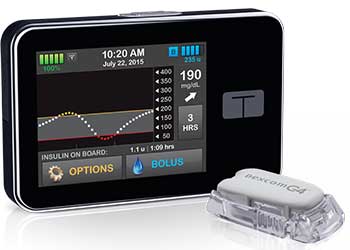
The medical device company, Tandem Diabetes Care, recently had their t:slim G4 insulin pump with Continuous Glucose Monitoring (CGM) Integration approved by the U.S. Food and Drug Administration (FDA). The t:slim G4 pump is the only pump that conveniently displays CGM graphs and trend information with current insulin delivery activity together on the home screen.
The pump features a touch-screen, built-in rechargeable battery, and a micro-USB port, all of which is expected to allow for a convenient and user-friendly experience. The CGM will be powered through the Dexcom G4 PLATINUM sensor, but the user will have the ability to use the pump without the CGM feature. Although small in size, the pump can hold up to 300 units of insulin and will allow the user to enter personal information and set glucose targets, alerts, and alarms.
“CGM integration has been one of the most requested features since the launch of the t:slim Pump. The addition of t:slim G4 to our product family allows us to address a wider variety of diabetes management needs through the broadest range of insulin pump options available from one company,” says Kim Blickenstaff, president and Chief Executive Officer of Tandem Diabetes Care.
Shipments for the pumps are expected to begin in October 2015.
Source: Tandem Diabetes Care, Inc.